Pool Water Chemistry
Understanding swimming pool water chemistry is an essential part to maintaining safe and consistent swimming pool operation.
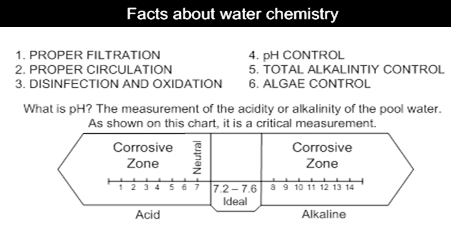
1. pH
The pH is one of the most important factors in pool water balance. The water€™s pH is a measure of its total acid alkalinity balance, the relative proportion of acids and alkalis in the water. The pH range goes from 0 14. i.e. on the pH scale, 0-6 level indicates acidity, 7 indicates a neutral state and 8-14 indicates alkalinity.
The ideal level for swimming pool water is between 7.2 and 7.8
Why is pH so important?
• You cannot run your pool at pH 6.5 - it would acidic enough to corrode the metal fittings in your pool circulation system and it is too far from the Human body's pH of 7.4 to be comfortable to bathe in. The compromise is 7.2 to 7.6, preferably midpoint of 7.4. Remember, if you let the pH drift out of this range, you will have to use more chlorine to get adequate disinfection.
• Bather comfort, at high pH, the water will make your eyes sting and possibly give you a sore throat.
• At high pH (above 8) there are two dangers.
- The danger of scale forming on your pool surfaces, pipe work and fittings. This is because at a pH of around 8.0, the calcium in the water combines with carbonates in the water. Result? --- Calcium carbonate or scale.
- Calcium carbonate can form into tiny particles and float around in the Water giving it a cloudy, turbid appearance.
- A low pH (below 7) can corrode metals, eating away at copper fittings and heat exchangers leaving metal oxides to stain pool surfaces. Under certain conditions the precipitated (particulate) metals can tint your hair,
2. Total Alkalinity
Total alkalinity is a measure of the amount of alkaline materials in the water. This alkalinity will usually be present as bicarbonates, but with a very high pH carbonates and hydroxides can be present as well.
In basic terms TA acts like a buffer for your pH level.
Total alkalinity is a measure of the pH-buffering capacity, or the water's resistance to a change in pH. This ability to resist change in pH is due primarily to the presence of the family of carbonate ions, but certain other compounds also provide buffering. The carbonate ions have a special role in water saturation. The operator must control both the amount of carbonate alkalinity and the pH to provide enough calcium carbonate to saturate the water without having so much that scale forms.
Alkalinity does not have to be tested for as often as pH. The good total Alkalinity will make it much easier to maintain good pH. The appropriate range for Total Alkalinity in pool water is between 75 and 120 ppm (parts per million). High Total Alkalinity (above 120 PPM) will allow your pH to slowly creep up and resist efforts to change.
Low Total Alkalinity (below 75 PPM) allows your pH to "bounce" from one extreme to the other, making it very difficult to keep your pH in the appropriate range. Ideal Total Alkalinity (between 75 and 120 PPM) can be achieved by adding Alkalinity Increaser if the Total Alkalinity is below 75 PPM and pH Reducer if Total Alkalinity is above 120 PPM now for the third factor affecting your swimming pool water chemistry.
3. Total Dissolved Solids
By definition, TDS is absolutely everything dissolved in your pool water, from metals to chlorine to alkalinity to sulfates and salts. This apparent contradiction in terms refers to conductive chemicals that can accumulate in the pool particularly when the water evaporates, or when the pool is not 'diluted' with sufficient fresh water.
You cannot see them because they are dissolved, but this doesn€™t stop those corroding metal parts (pumps, pipe work, filters) on account of their conductivity. They are mostly made up of chlorides and sulphates.
The acceptable range of TDS in a swimming pool is between 1,000 and 2,000 ppm.
4. Calcium Hardness
Water can be corrosive even if the PH and total alkalinity levels are in the proper range. The reason is water has a natural demand for calcium. The recommended range of Calcium Hardness is 200-275 ppm
5. Temperature
Temperature is the property that gives physical meaning to the concept of heat. If an object is cold, we say it has a low temperature. If it is hot, we say it has a high temperature. It can also be observed that if a hot poker is plunged into cold water, the poker becomes cooler and the water becomes warmer. This means that the hot body gives up some of its heat to the cold body.
6. Turbidity
Turbidity is important in terms of the clarity of the swimming pool water. Water should be maintained to a level which will ensure the safety of bathers. It is vitally important that the bottom of the pool is always visible so that a person coming to rest at the deepest point of the pool is seen. It should be possible to see the bottom of the pool clearly from any position on the pool side when the pool is in use. The pool should be closed whenever this standard fails to be achieved.
7. Free Available Chlorine
The disinfectant form of chlorine is "free residual chlorine." It is also known as €œfree available chlorine€ or €œfree chlorine€ and all terms refer to the concentration of hypochlorous acid and the hypochlorite ion in equilibrium concentration in the pool water. It is strong and safe when used properly and is still the most popular form of disinfection.